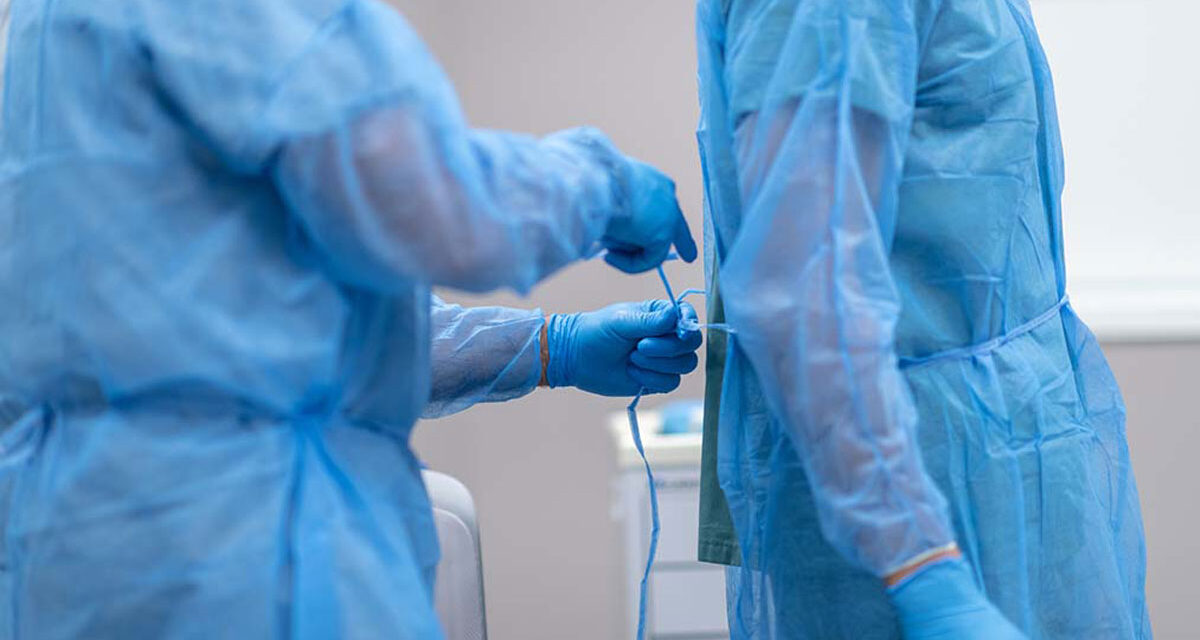
COVID-19 has put a critical focus on infection control and personal protective equipment (PPE) as Senior Living communities seek to protect residents, staff and visitors. Choosing PPE gowns, or isolation gowns, can be tough during conventional capacity times when PPE is in full supply. During crisis capacity times, the decision can be even more difficult with the need for dozens of isolation gowns per day or even per shift. Here are some key questions and considerations.
What Are Isolation Gowns?
Isolation gown purpose is to help protect your frontline caregivers and staff from infectious droplets, fluid penetration and solids, and help prevent the transfer of micro-organisms to vulnerable residents.
There are two main categories of isolation gowns intended for healthcare purposes: surgical and non-surgical.
- A surgical gown is a personal protective garment intended to be worn by health care personnel during surgical procedures to protect both the patient and health care personnel from the transfer of microorganisms, body fluids, and particulate matter.
- Non-surgical, or isolation, gowns are Class I devices (exempt from premarket review) intended to protect the wearer from the transfer of microorganisms and body fluids in low or minimal risk patient isolation situations. Non-surgical gowns are not worn during surgical procedures, invasive procedures, or when there is a medium to high risk of contamination.

Know the Level of Protection Standards
The FDA recognizes the consensus standard American National Standards Institute/Association of the Advancement of Medical Instrumentation (ANSI/AAMI) PB70:2003, “Liquid barrier performance and classification of protective apparel and drapes intended for use in health care facilities.”
There are four levels under the standard:1
- Level 1: Minimal risk, to be used, for example, during basic care, standard isolation, cover gown for visitors, or in a standard medical unit.
- Level 2: Low risk, to be used, for example, during blood draw, suturing, in the Intensive Care Unit (ICU), or a pathology lab.
- Level 3: Moderate risk, to be used, for example, during arterial blood draw, inserting an intravenous (IV) line, in the emergency room, or for trauma cases
- Level 4: High risk, to be used, for example, during long, fluid intense procedures, surgery, when pathogen resistance is needed or infectious diseases are suspected (non-airborne)
For Senior Living, Level 1 basic fluid resistance is generally desirable to combat the spread of COVID-19. Having a gown with a higher level rating isn’t essential as there isn’t extensive risk of blood or other bodily fluids being transferred. For more advanced fluid-resistance needs, consider a surgical gown with a higher-level rating.
What’s on the Product Label is More Important Than the Product Name
For these purposes, we use the term isolation gown. But you should pay less attention to a product name (e.g., isolation gown, nursing gown, procedural gown, etc.) and more attention to function, intended use and what level of protection is provided. The label or packaging will call this out.
During the COVID-19 pandemic, primary objectives are to protect frontline Senior Living staff from the spread of COVID-19 and to protect the accidental transfer of COVID-19 to other residents and staff. Level 1 basic fluid resistance in a non-surgical isolation gown is most likely sufficient. Having a surgical gown with a higher level rating isn’t essential as there isn’t extensive risk of blood or other bodily fluids being transferred.
Evaluate Purpose, Material and Clean vs. Sterile When Choosing Isolation Gowns for Healthcare Settings
The Centers for Disease Control suggests that you consider three things when choosing gowns for healthcare settings.2
Purpose
During the COVID-19 pandemic, primary objectives are to protect frontline Senior Living staff from the spread of COVID-19 and to protect the accidental transfer of COVID-19 to other residents and staff. Level 1 basic fluid resistance is often sufficient.
Material
What are isolation gowns made of? Typically cotton or a synthetic material like polyester (reusable isolation gowns), or polyethylene or polypropylene (disposable gowns). They can also be latex-free. Synthetic materials are generally better at blocking fluids and are preferred over cotton to prevent the spread of COVID-19.
Clean vs. Sterile
Clean isolation gowns are used for isolation, while sterile gowns are used for invasive procedures like inserting a central line. For COVID-19, a clean isolation gown works well.
How Easy is an Isolation Gown to Put On and Remove?
The ease or difficulty with which a gown is put on and removed may affect its effectiveness and the potential for self-contamination, especially during the doffing of a contaminated gown.
How Many Different Sizes and Fits Do You Need?
In a non-COVID-19 world, each staff member would have a gown that fit them perfectly. During the current PPE shortage, that’s not realistic in many parts of the country, and a universal size may be the only option. In such cases it’s still critical to make sure that the gown allows the wearer enough freedom of movement to perform their required tasks while still providing as much coverage of their skin and clothing as possible.
Isolation Gown FAQ:
What are isolation gowns?
There are two main categories of isolation gowns intended for healthcare purposes: surgical and non-surgical.
What are isolation gowns made of?
Typically cotton or a synthetic material like polyester (reusable isolation gowns), or polyethylene or polypropylene (disposable gowns).
What are PPE gowns used for?
Clean isolation gowns are used for isolation, while sterile gowns are used for invasive procedures like inserting a central line.
Looking for Isolation Gowns?
Direct Supply is your source for a wide variety of PPE, including:
Rely on our expertise, selection and service to help you find exactly what you need to protect your communities, residents, staff and visitors. You can also visit our dedicated COVID-19 resources page or call 800-634-7328.
References
1 “Medical Gowns | FDA.” U.S. Food and Drug Administration, FDA, https://www.fda.gov/medical-devices/personal-protective-equipment-infection-control/medical-gowns. Accessed 10 July 2020.
2 “Guidance for the Selection and Use of Personal Protective Equipment (PPE) in Healthcare Settings.” Centers for Disease Control & Prevention, Centers for Disease Control & Prevention, https://www.cdc.gov/HAI/pdfs/ppe/PPEslides6-29-04.pdf. Accessed 10 July 2020.